
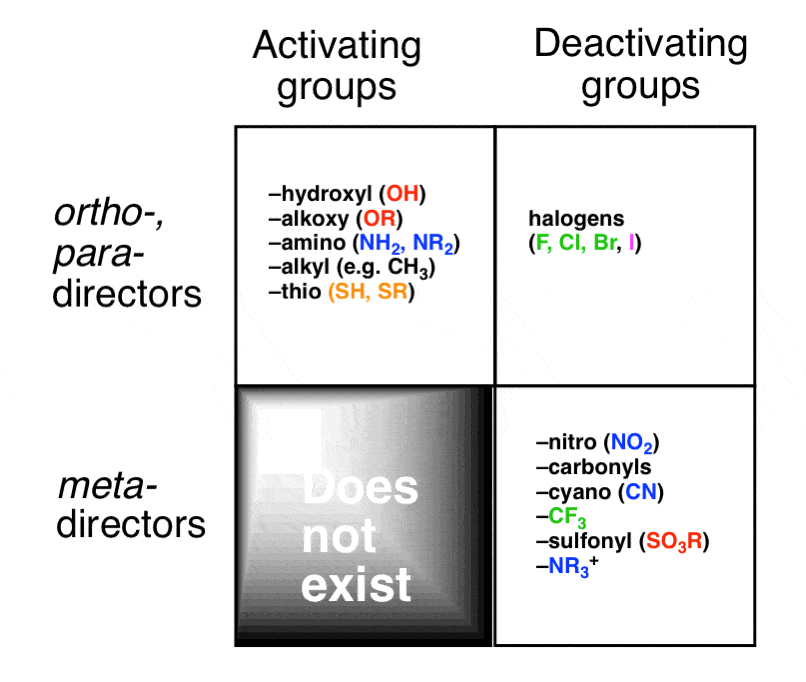
This video takes you through the logic and structure of the ortho and para directing activating groups. Now that you've mastered resonance, let's take a look at the directing effects of activating and deactivating groups. There's the long way, drawing resonance structures, and the short way using my awesome shortcut.Īctivating Groups are Ortho/Para Directors. The key to mastering directing effects of activating and deactivating groups lies in understanding the nature of the sigma complex resonance intermediates. Video 10 – Resonance and Trick for Ortho Meta and Para Addition Intermediates Video 9 – Ortho Meta and Para Disubstituted Benzene Monster TrickĬonfused about Ortho, Meta, and Para? Let the monster in the video below help you remember! This video shows my fun trick for easily recognizing ortho, meta, and para substituents on a disubstituted benzene. To learn more, read about Ortho, Meta, Para Directing Effects in EAS reactions here.

Another way to look at them is to see if they direct the incoming groups to the Ortho, Meta, or Para positions. Substituents on benzene will fall into 2 main categories – activators and deactivators. When it comes to a substituted benzene, the chemistry and nature of the existing substituent (or substituents) will determine not only where the next group adds onto the ring, but also if the group is allowed to add at all. ◊ Directing Effects On Substituted and Multi-Substituted Benzene ◊
#ELECTROPHILIC AROMATIC SUBSTITUTION REACTIVITY HOW TO#
This video shows you a comparison of the FC alkylation and acylation reactions, including the limitations of FC alkylation, and how to convert an acylation product to the reduced alkyl version. Video 8 – EAS Friedel-Crafts Alkylation vs Acylation This video shows you the mechanism for the formation of Nitronium – the super electrophile that is attacked by benzene in the nitration reaction. This video shows you the aromatic halogenation mechanism from the role of the Lewis Acid catalyst and formation of the super-electrophile, through the entire mechanism of adding halogen to benzene.Īdding a nitro group (NO2) to the benzene ring. The most common EAS reactions you will cover are as follows: Video 3 – EAS Aromatic Halogenation The next few videos will take you through the most common EAS reactions, focusing on the mechanism for the super-electrophile creation as well as the actual addition to benzene. While many of your exam questions will focus on adding groups to a monosubstituted or disubstituted benzene, it's important that you first get a solid understanding of the basics. ◊ Basic EAS reactions adding to Benzene ◊ However, the intermediate does have some stability due to its ability to resonate the 2 remaining pi bonds on the sigma complex.

Since benzene rings are aromatic, they are less than thrilled to ‘break open' and undergo an EAS reaction. Video 2 – EAS Mechanism + Sigma Complex Resonance The EAS Intro video below gives you a detailed overview of the EAS reaction, along with a comparison to alkene addition reactions and the need for a Super-Electrophile Video 1 – Introduction to Electrophilic Aromatic Substitution Electrophilic Aromatic Substitution Cheat Sheet.Halogens are exceptions – Deactivating Ortho/Para Directors.Activating Groups – Ortho/Para Directors.Ortho/Meta/Para – ‘Monster' Trick Video, Shortcut for Substituted Benzene.Directing Effects on Substituted and Multi-Substituted Benzene:.Friedel-Crafts – Alkylation, Acylation, Comparison of Alkylation vs Acylation Reactions.Aromatic – Halogenation, Nitration, Sulfonation.


 0 kommentar(er)
0 kommentar(er)
